The joint research team of Professor Hee-Tak Kim, from the Department of Chemical and Biomolecular Engineering, and Shin-Kun Ryi, PhD, from the Korea Institute of Energy Research Advanced Materials and Devices Laboratory, has announced a new production method for vanadium redox flow batteries (VRFBs) that reduces costs by 40%. The research paper, titled “Catalytic production of impurity-free V3.5+ electrolyte for vanadium redox flow batteries”, was published in Nature Communications on September 27 and earned a spot in the “Editors’ Highlights” section.
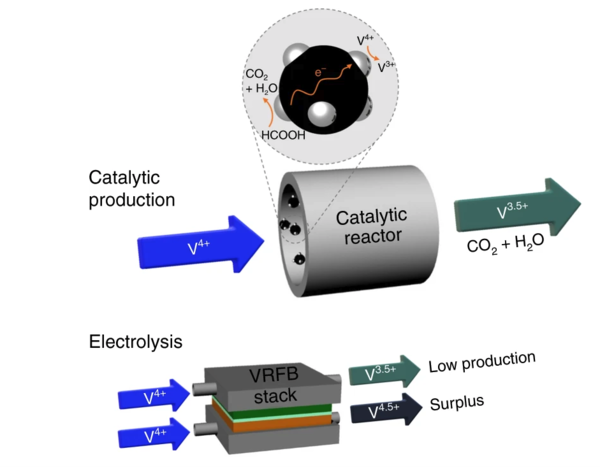
With increasing concerns over the safety of lithium-ion batteries and their inflammable nature, attention has been brought to batteries that use aqueous electrolytes, which are inherently not flammable. While VRFBs are a suitable alternative due to their durability, safer nature, and ability to store large amounts of energy, relatively higher costs has stagnated their entry into the commercial market. The electrolyte, which is responsible for the battery’s capacity and lifespan, is the main culprit, accounting for around 40-50% of the production cost.
The conventional way of producing the electrolyte consists of two steps. While the first step is easily conducted via a chemical reduction reaction with a residue-free organic reducing agent (ORA), the second step is more sluggish when using the same method. Although an electrolysis reaction is used as an alternative, such a method is very complex, requiring costly devices and an additional reduction of surplus electrolytes.
While many research projects have focused on using a chemical reaction, the residue from the reactions left the electrolytes contaminated. However, the newly developed method by the joint research team uses catalysts to speed up chemical reactions with ORAs, which intrinsically have low reactivities. The reaction uses formic acid as the ORA and Pt nanoparticles supported on carbon black (Pt/C) as the catalyst and only produces water and CO2 as byproducts, which are easy to remove.
Through the newly developed method, the research team successfully produced pure electrolytes at a rate of two liters per hour, similar to the output of the electrolysis method but at a much lower cost. Ryi hoped that “the newly developed catalytic reaction can promote the industrialization of the [VRFB] technology.”

