Solar energy has gained popularity as a new, environmentally-friendly source of energy to replace fossil fuels; Solar energy reduces the greenhouse gases in the atmosphere by converting carbon dioxide into methane. However, the conversion has a rate too low for practical use. The catalysts used are based on expensive platinum, and carbon dioxide is too stable for a high conversion rate even with such catalysts.
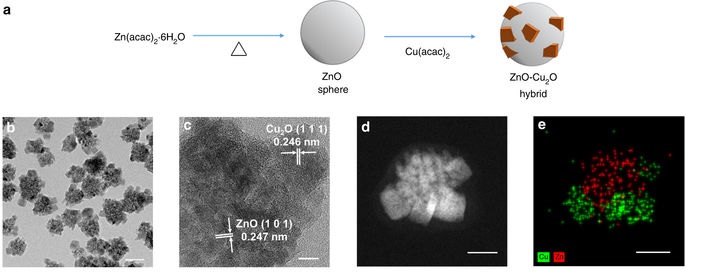
Professor Hyunjoon Song of the Department of Chemistry, along with Professor Ki Min Nam from Mokpo National University, has developed a metal oxide hybrid optical nanocatalyst in order to increase the methane conversion rate with relatively cheap materials. The research team synthesized zinc oxide nanoparticles and then coated these with copper oxide to form a colloid-type zinc- copper oxide hybrid nanostructure. The results of the research were published in the journal Nature Communications on November 7.
The new structure maintained the reaction for a longer period of time than the conventional reactions in aqueous mediums. Both copper oxide and zinc oxide generate electrons when exposed to sunlight and convert carbon dioxide into methane. The new hybrid structure allowed the zinc oxide to pass the generated electrons to the copper oxide at the surface, increase the overall duration of the reaction. As a result, 99 percent pure methane was produced from carbon dioxide through the medium.
The reduced size of the particles greatly increased the conversion rate. The conventional photocatalysts are not uniform and exist in large chunks. By reducing the size of the particles down to the nanoscale and binding the two oxides together, the research team managed to increase the surface area significantly. This resulted in a hundred-fold increase in carbon dioxide conversion activity in aqueous solutions than that of conventional catalysts.
Professor Song commented, “The commercialization of methane conversion will take time as it is in its primitive stage. However, we hope the catalyst can be applied to other photocatalytic reactions.”