A research team led by Professor Yousung Jung from the Department of Chemical and Biomolecular Engineering elucidated the mechanism by which single-substance catalysts with complex surface morphology act in the hydrogen evolution reaction (HER). The research was carried out in collaboration with Professor Xiangfeng Duan from UCLA and Professor William A. Goddard III from California Institute of Technology. The proposed mechanism sheds light on how controlling morphology of the platinum catalyst can increase activity and optimize the chemical process.
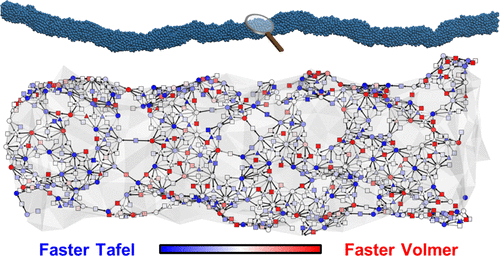
Platinum metal has important applications as a catalyst in the chemicals industry, used in processes such as the electrolysis of water to obtain hydrogen gas and in fuel cells that can power electric cars. However, its high cost has been a limiting factor to expanding the use of the catalyst outside of labs. Although researchers found that the efficiency of platinum catalysts could be substantially improved by shaping them into jagged nanowires, the reason for the higher mass activity resulting from a change in the catalyst morphology remained unknown.
Previously established mechanisms for the role of a catalyst in the HER suggested that the two main types of reactions required for hydrogen evolution from water, electrochemical proton adsorption and activation by coupling reactions between adsorbed hydrogen atoms, occur on the same reaction site. However, Professor Jung’s team analyzed jagged platinum nanowires using deep learning methods that predict surface properties of catalysts to suggest otherwise. They concluded that when platinum has an uneven structure, some reaction sites on the surface prefer adsorption while others are specialized for activation. This resulted in the platinum acting like a combination of two catalysts, and a four-fold increase in the activity was observed.
Through these novel insights, single-substance catalysts can be designed to further improve the economics of many chemical processes. It is also expected that the reduction of energy required to drive these processes will aid the movement toward sustainable energy — larger amounts of renewable hydrogen may be produced and used as an alternative to fossil fuels. The new findings were published on March 17 in the Journal of the American Chemical Society under the title “Autobifunctional Mechanism of Jagged Pt Nanowires for Hydrogen Evolution Kinetics via End-to-End Simulation”.

