Trying to extend the human lifespan is a struggle as old as civilization. Modern studies in the aging of the human body differentiate between life span, which is the time from birth until death, from health span — the period of time for which one is in good health. Extending age while minimizing risk of age-susceptible diseases and disabilities is incredibly complex, but KAIST researchers may have had a large breakthrough in identifying a single amino acid change that could make all the difference.
A research team led by Professor Seung-Jae V. Lee from the Department of Biological Sciences found that a single amino acid change in the protein phosphatase and tensin homolog (PTEN) was able to significantly extend the health span of their roundworm (C. elegans) test subjects. PTEN is a phosphatase, which removes phosphate groups from lipids and proteins. It is one of the key proteins in cell cycle regulation, which means it is one of the body’s best defense mechanisms against tumors. Since tumors are one of the most common forms of age-related diseases, the research team found evidence of the importance of this protein in extending the health span of organisms.
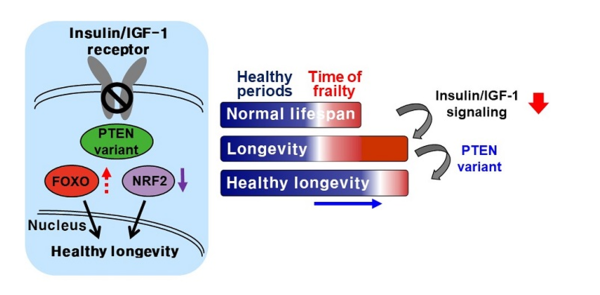
Another factor in longevity in organisms is Insulin and Insulin-like growth factor-1 Signaling (IIS). This is a pathway in organisms that control the organism’s aging and life span, and many studies have found that a significant increase in longevity occurs in organisms when this process is suppressed. However, this isn’t without consequences — if the process is suppressed too much, health defects including processes related to reproduction and growth become more common.
Professor Lee’s research team found that a single amino acid change in PTEN improves health status significantly through cell cycle regulation, while maintaining the extended longevity from the suppression of IIS. A notable observation recorded by the research team was that after the single amino acid was modified, PTEN preferentially maintained protein phosphatase activity, whilst reducing lipid phosphatase activity. This was seen as a recalibration of the IIS process. By changing the singular amino acid, the activity of the transcription factor Forkhead Box O (FOXO) was maintained, allowing longevity promotion. Simultaneously, Nuclear factor-erythroid factor 2-related factor 2 (NRF2), a regulator for antioxidant and xenobiotic defense, was suppressed. This prevented the reduction in IIS from causing health defects in organisms with extended life spans.
Although the tests have only been conducted on C. elegans so far, Professor Lee is optimistic in his view that the team’s study “raises the exciting possibility of simultaneously promoting longevity and health in humans”. This research was supported by the Ministry of Science and ICT through the National Research Foundation of Korea.

