As the global battery market size is growing exponentially, researchers around the world have been putting much effort into increasing the efficiency and stability of rechargeable batteries. In an interview with The KAIST Herald, Ph.D. student Jinha Jang from the Department of Materials Science and Engineering introduced his paper titled “Self-Assembled Protective Layer by Symmetric Ionic Liquid for Long-Cycling Lithium-Metal Batteries” published in Advanced Energy Materials in February, sharing its key points and the inside story.
Please briefly introduce yourself to our readers.
My name is Jinha Jang. I am in my third semester of the Ph.D. program under Professor Jiheong Kang’s lab. Recently, I led the joint research with Professor Kang and Professor Chan Beum Park from the Department of Materials Science and Engineering.
Could you explain your scientific paper to the readers?
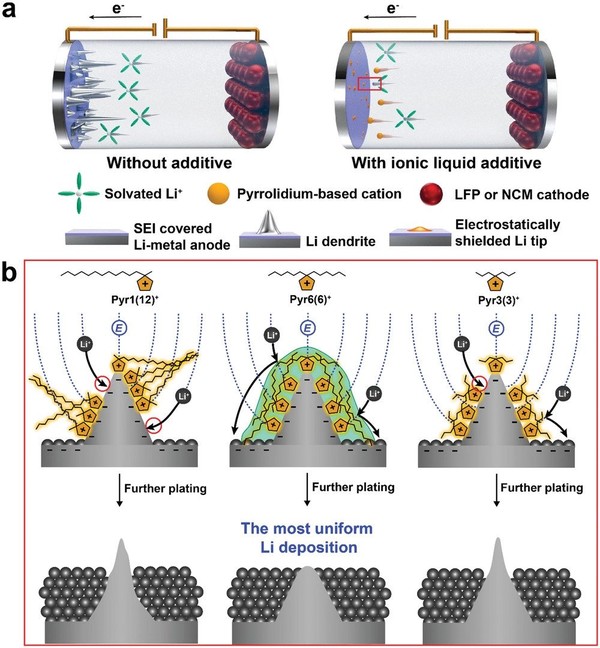
Before I start talking about the paper, I have to talk about lithium metal batteries. A lithium metal battery replaces conventional graphite cathodes with lithium metal cathodes and is a next-generation battery with a higher energy density than conventional batteries. However, lithium metal has a problem in that lithium dendrite generated during deposition causes an internal short circuit, thereby degrading the battery life and stability. This needle growth occurs when the lithium ion flow is concentrated on the protrusion compared to the flat part due to the strong electromagnetic field.
Therefore, ionic liquid additives have been used to suppress such phenomena. The cations of the ionic liquids are adsorbed to lithium tips to form an alkyl chain-based lithiophobic protective layer, inducing uniform lithium deposition. However, conventional ionic liquids such as pyrrolidinium 1(12) (Pyr1) have an asymmetric molecular structure and show high amphiphilic properties, resulting in self-aggregation. Therefore, we have introduced symmetric alkyl chain-based ionic liquids to alleviate polar properties, forming a uniform anti-lithium protective layer without aggregation of ionic liquid.
In an attempt to make batteries stable, you used different ionic liquid additives. Why and how did you design these ionic liquid additives?
The main [goal] was to prevent the self-aggregation phenomenon. So, we conducted the experiment simply thinking that if we synthesize alkyl chains that have similar molecular sizes to Pyr1 but are spread out evenly on both sides, we can create a more uniform anti-lithium protective layer. Therefore, we selected pyrrolidinium 6(6) (Pyr6) as the experimental group. Pyrrolidinium 3(3) (Pyr3) with a molecular size smaller than Pyr6 was also designed to observe the effect of the symmetric alkyl chain length. The results showed that the battery life stability of Pyr6 was significantly higher than that of the other two ionic liquids, which means that n-hexyl chains are the most effective in forming uniform anti-lithium protective layers among symmetric alkyl chains.
To put it simply, the commercially used liquid electrolyte currently, LiTFSi, has a capacity retention of less than 40% at about 200 cycles, while the use of the Pyr6 electrolyte additive has a capacity retention of 80% at 600 cycles and shows 99.6% coulomb efficiency per 30,000 batteries.[Using Pyr6, the battery] has more than three times the battery cycle stability compared to the conventional commercial electrolytes.
Will this research result be applied to the battery industry soon?
Many people wondered about the commercialization of this new technique. We plan to patent this research result in cooperation with LG Energy Solution. This week, we decided to apply this new ionic liquid to lithium metal batteries and lithium sulfur batteries used in the industry. If the result comes out positive, technology transfer may be possible.
Were there any challenges while conducting this study, and if so, how did you overcome them?
I did not take any electrochemistry courses or conduct related research both during my undergraduate and my master’s degree programs. I did not know much about batteries. I think the first few months of my Ph.D. program were the hardest period when I studied about the battery. However, I was able to quickly grasp the basic knowledge through corporate homepage materials, videos, and scientific papers. The habit of organizing papers in the PowerPoint format also helped me understand the trend of battery research well.
How did you become interested in the field of batteries?
With the recent development of advanced technologies such as unmanned aerial vehicles and electric vehicles, research on the development of more efficient energy storage materials is of great interest. I was swept away in the moment and became interested in this field.
Is there any research you want to do in the future?
We already started the next research — it is about polymer electrolytes. Polymer electrolytes have the advantage of being more flame-retardant and electrochemically safer than liquid electrolytes, whereas the main drawback is their low ion conductivity. We are developing a new type of macromolecular polymer electrolytes that overcome this limitation. It will function as a separator and serve to transfer lithium ions.

