While cancer has been a disease unconquered by modern medical science, one of the areas of interest in cancer treatment is utilizing the immune system of cancer patients. The KAIST Herald interviewed Sangjoon Lah, a PhD student from the Department of Biological Sciences who published a paper titled “Engineering second-generation TCR-T cells by site-specific integration of TRAF-binding motifs into the CD247 locus”. The paper was featured in the Journal for ImmunoTherapy of Cancer on April 5.
Can you explain your scientific paper to our readers?
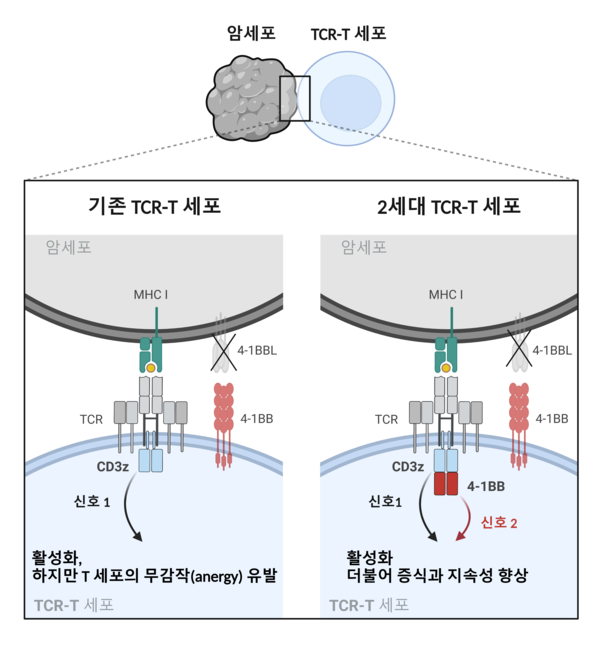
We established second-generation TCR-T cell therapy using CRISPR-Cas9 gene editing technology. [Our new technology incorporates] a co-stimulatory domain called the TRAF2-binding domain into the CD247 gene, a critical component involved in T cell receptor (TCR) signaling. We confirmed that these second-generation TCR-T cells exhibited superior anti-tumor effects compared to conventional TCR-T cells in the xenograft mouse model of malignant melanoma.
What was the initiative for this research?
The development of second-generation chimeric antigen receptor (CAR)-T therapy engineered to include co-stimulatory signals has yielded remarkable clinical results, with complete remission rates of over 80% observed in refractory or relapsed B-cell leukemia patients. [Likewise], we expect that incorporating co-stimulatory signals into TCR-T cell therapy, which primarily targets solid tumors, will enhance its anti-tumor efficacy.
Did you face any difficulties while conducting the research? How did you overcome them?
Compared to CARs, which are made up of single-protein receptors, TCRs are multi-protein complex receptors. Thus, engineering TCRs to include co-signaling domains is a much more challenging task. We designed numerous potential candidate receptors and, in fact, after many trials, we were ultimately able to find the most suitable form.
What is the significance of your research in the scientific field and our lives?
My research has provided valuable insight into the development of the next generation of TCR-T cell therapy, which has shown great potential as a cell-based cancer therapy. This promising approach could play a crucial role in the treatment of solid tumors, offering new hope for cancer patients.
Is there any follow-up research you want to conduct in the future?
The immunosuppressive characteristics of the tumor microenvironment and the presence of intratumoral heterogeneity are two major obstacles to the success of cancer therapies. Currently, I am conducting a follow-up study focused on exploring novel strategies aimed at enhancing the immunity against the immunosuppressive tumor microenvironment. In addition, I am also investigating the potential of developing a multi-targeted system to effectively overcome tumor heterogeneity.

